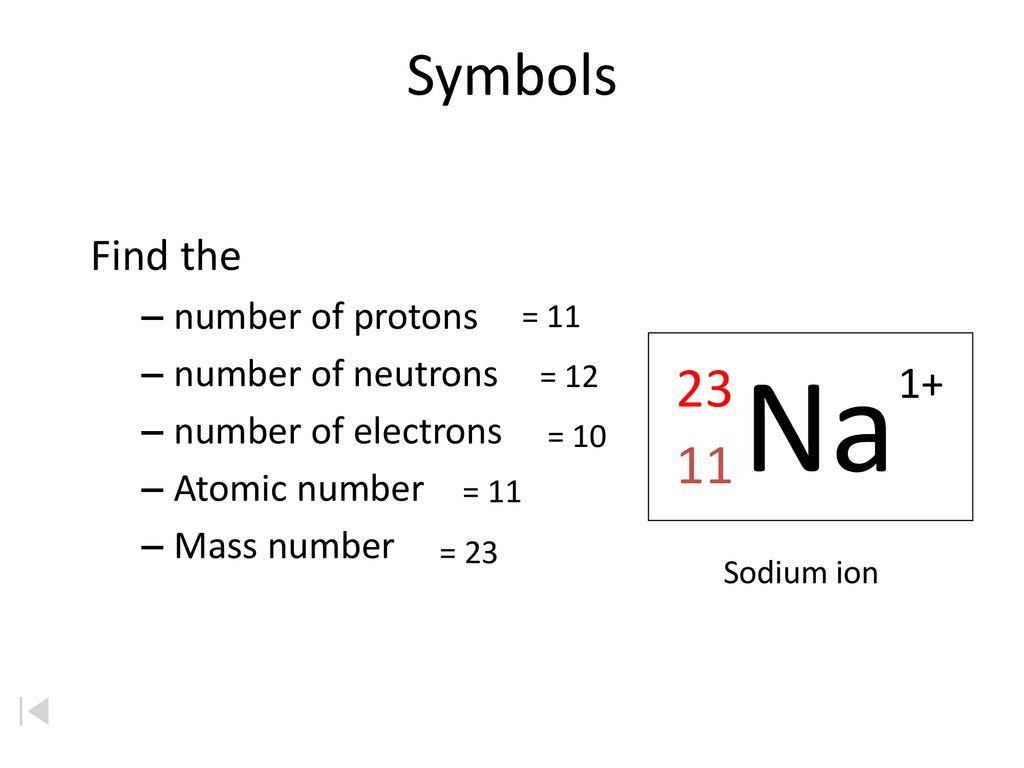Ace Tips About How To Find Out Mass Number

Atomic mass is not reported with units.
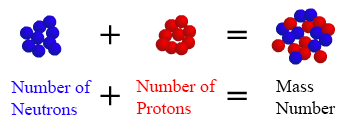
How to find out mass number. All atoms of a given. The sum of the number of neutrons and protons is known as the mass number. (atomic mass of each isotope) x (%abundance /100) 34.96885*0.7578 = 26.50 (i) 36.96590*0.2422 = 8.95 (ii) step 2:
School chemistry2020 youtube channel carrying chemistry and science subjects, current affairs related videos post only.i will explain how to find out mass nu. Atomic mass = mass of protons + mass of neutrons + mass of electrons. While mass is defined by f = ma, in situations where density and volume of the object are known, mass is also commonly calculated using the following equation, as in the calculator provided:.
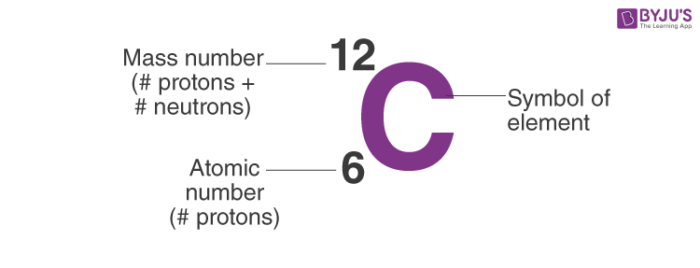
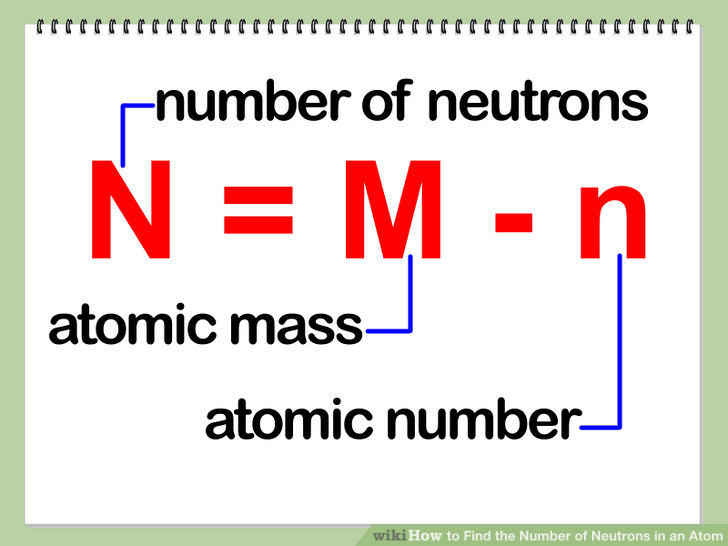
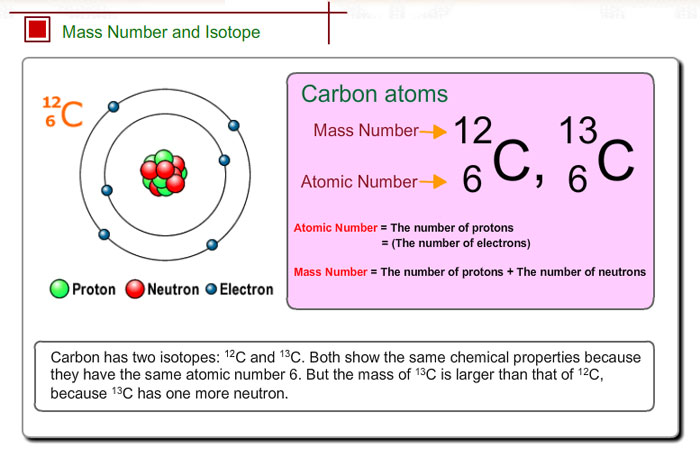
Number of protons = atomic number number of electrons = atomic number number of. Mass number is measured in atomic mass units and is represented with the symbol a ; The number of protons in an atom of an chemical element is its atomic number.
Adding (i) and (ii), the atomic mass of the given sample is. To find the atomic mass of a given element or an isotope of an element, there are 3 steps involved: Lesson for chemistry i, general chemistry i, and honors chemistry i.
The element is arsenic (as). The atomic number of arsenic (as) is 33. Atomic number and mass number diminutive number.
Method 1 sum total of protons and neutrons of a single atom these steps. How to interpret atomic numbers and mass numbers to calculate the number of neutrons in. Find the number of protons of the element from the periodic table.